- Overview Product family
- SMART OR® Software Suite
- iOi Encoder and Decoder
- DOORSIGN Digital LCD terminal
- OPERION® AIO, integration into operating theaters, large screen, monitor
- CLINIO® C All-in-One PC
- CLINIO® D Medical Monitor
- SILENIO® D Medical Monitor
- SILENIO® C All-in-One PC
- MEDIGENIC® Equipment
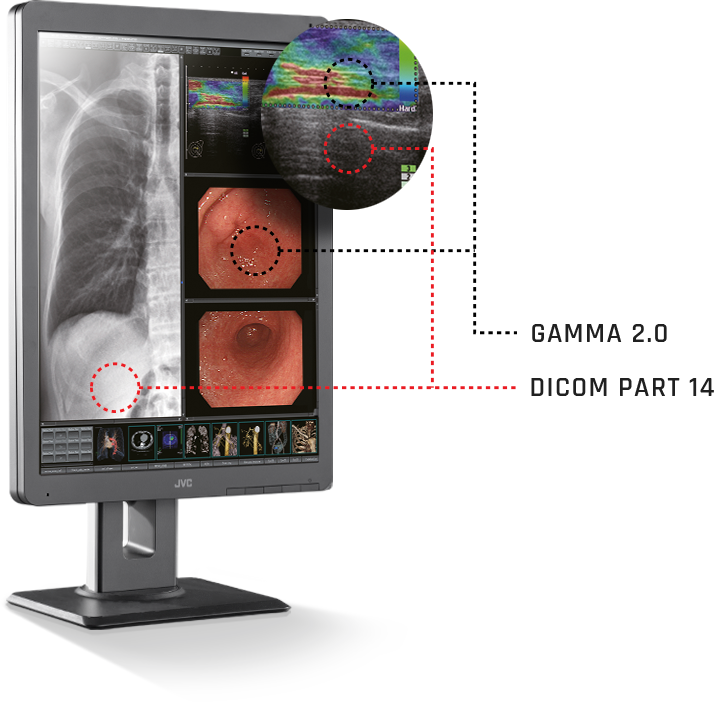
- JVC Displays Diagnostic monitors
- Service and Support Service Packages
-
Germany
+49 2161 / 6984 - 0
info@reinmedical.com -
Swiss
+41 71 / 929 55 99
info.ch@reinmedical.com -
Spain
+34 91 / 530 88 24
info.es@reinmedical.com